2022, 19 (4)

2025, 125 (18)

2021, 7 (48)

2023, 145 (15)

2021, 60 (13)

2023, 62 (41)
Featured Publications
Click for the full publication list: Google Scholar
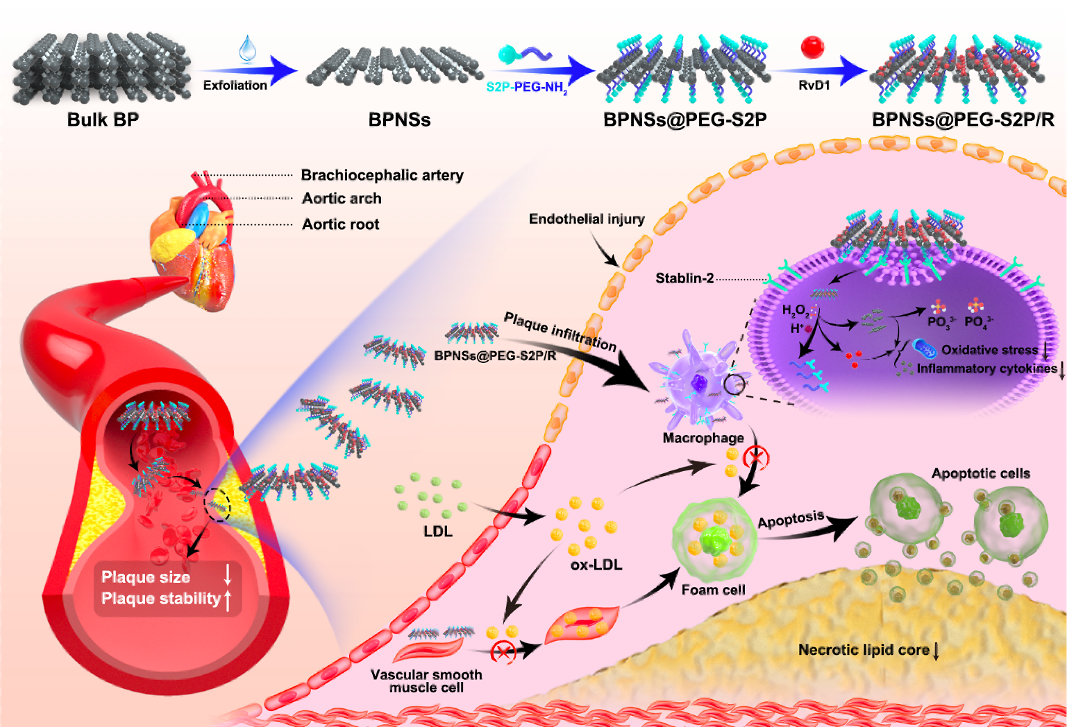
Resolvin D1 delivery to lesional macrophages using antioxidative black phosphorus nanosheets for atherosclerosis treatment
Current systemic anti-inflammatory therapies for atherosclerosis are limited by off-target adverse effects and insufficient efficacy within advanced plaques, as exemplified by large-scale clinical trials such as CANTOS and CIRT, motivating the development of macrophage-targeted and lesion-specific interventions.
In this study, we demonstrate the potential of black phosphorus nanosheets (BPNSs) as a therapeutic agent for the treatment of atherosclerosis. BPNSs can effectively scavenge a broad spectrum of ROS and suppress atherosclerosis-associated pro-inflammatory cytokine production in lesional macrophages. We also demonstrate ROS-responsive, targeted-peptide-modified BPNS-based carriers for the delivery of resolvin D1 (an inflammation-resolving lipid mediator) to lesional macrophages, which further boosts the anti-atherosclerotic efficacy. The targeted nanotherapeutics not only reduce plaque areas but also substantially improve plaque stability in high-fat-diet-fed ApoE-/- mice. [link]
Nature Nanotechnology 2024, 19(9):1386-1398.
(Highlighted in the ”Biomedical Engineering" Series of articles from across Nature Portfolio)
Macrophage-Modulating Nanosheets in Atherosclerosis
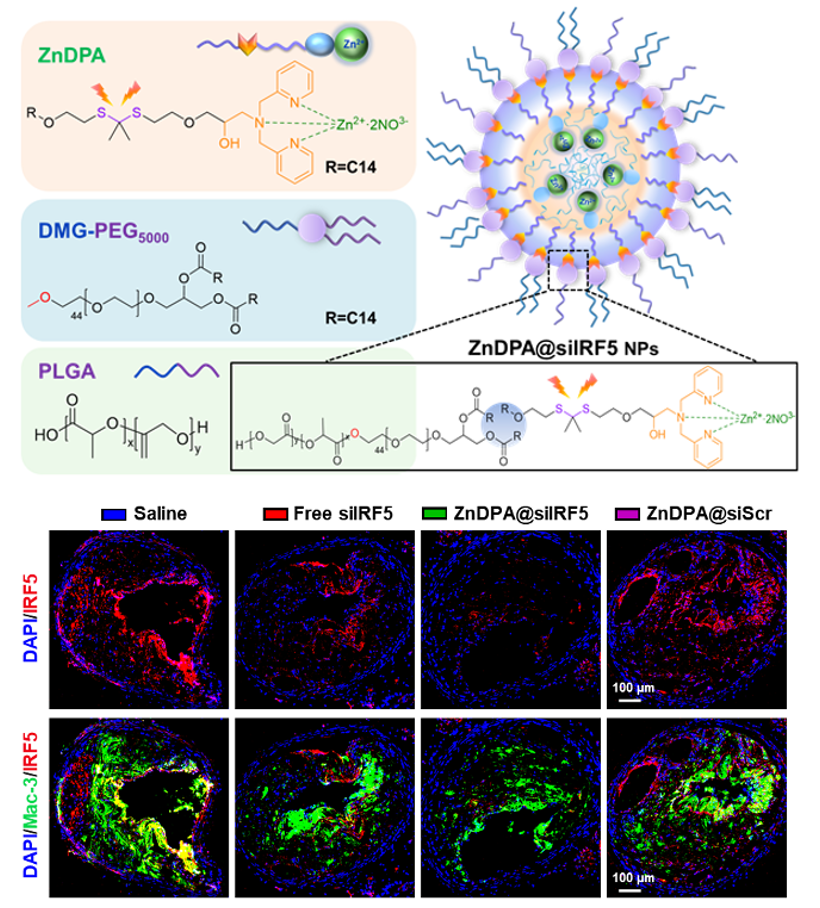
IRF5 siRNA Nanoimmunotherapy: Restoring Macrophage Efferocytosis in Atherosclerosis
Impaired efferocytosis of macrophages within advanced atherosclerotic plaques contributes to apoptotic cell accumulation, plaque necrosis, and eventual plaque rupture, ultimately leading to atherothrombotic events. Although restoring efferocytic capacity in lesional macrophages represents a promising therapeutic strategy, current approaches—such as anti-CD47–based regimens—are limited by off-target clearance of healthy tissues and associated toxicities, including anemia.
In this study, we developed an engineered small interfering RNA (siRNA) nanoparticle platform that can therapeutically manipulate lesional macrophages by inhibiting an overexpressed plaque-destabilizing macrophage molecule: IRF5. IRF5 siRNA (siIRF5) nanoimmunotherapeutics were efficiently taken up by lesional macrophages, particularly Cd11c+ and Trem2hi macrophages, and enhanced their phagocytic clearance of apoptotic cells by efficiently silencing IRF5 expression within these macrophage subsets in atherosclerotic plaques. This resulted in remarkable therapeutic efficacy, as evidenced by reduction of necrotic core area and enhancement of plaque stability in 2 independent ApoE−/− murine models of atherosclerosis. These findings highlight the potential of siRNA nanoimmunotherapeutics for treating atherosclerosis and other diseases resulting from impaired efferocytosis in macrophages. [link]
Circulation 2025, 152(22):1564-1581.
siRNA-mediated Nanoimmunotherapy in Atherosclerosis
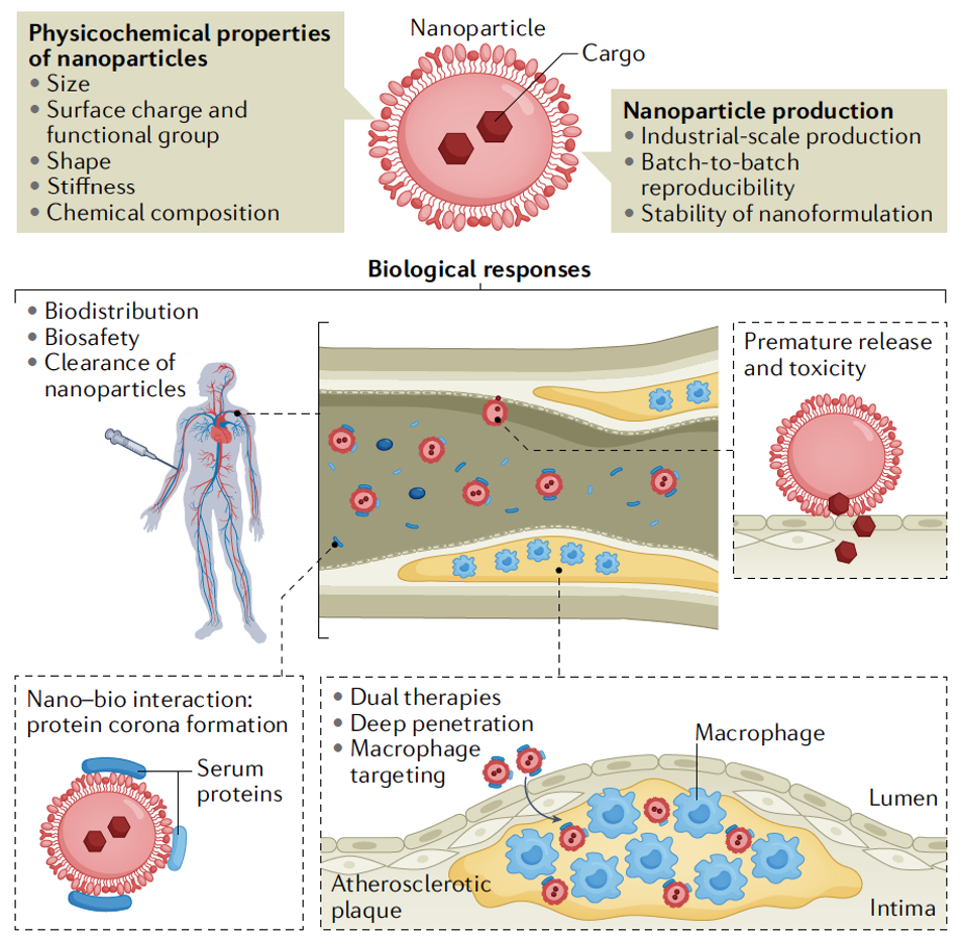
Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis
Nanotechnology could improve our understanding of the pathophysiology of atherosclerosis and contribute to the development of novel diagnostic and therapeutic strategies to further reduce the risk of cardiovascular disease. Macrophages have key roles in atherosclerosis progression and, therefore, macrophage-associated pathological processes are important targets for both diagnostic imaging and novel therapies for atherosclerosis.
In this Review, we provide novel perspectives on how macrophage-targeting nanoparticles can deliver a broad range of therapeutic payloads to atherosclerotic lesions. These nanoparticles can suppress pro-atherogenic macrophage processes, leading to improved resolution of inflammation and stabilization of plaques. Additionally, we propose future opportunities for novel diagnostic and therapeutic strategies and provide solutions to challenges in this area for the purpose of accelerating the clinical translation of nanomedicine for the treatment of atherosclerotic vascular disease. [link]
Nature Reviews Cardiology 2022. 19(4):228-249.
(Highlighted as Front Cover Paper; Highlighted in the "Mechanisms of atherosclerosis" Series of articles from Nature Reviews Cardiology)
Macrophage-Targeted Nanomedicine in Atherosclerosis
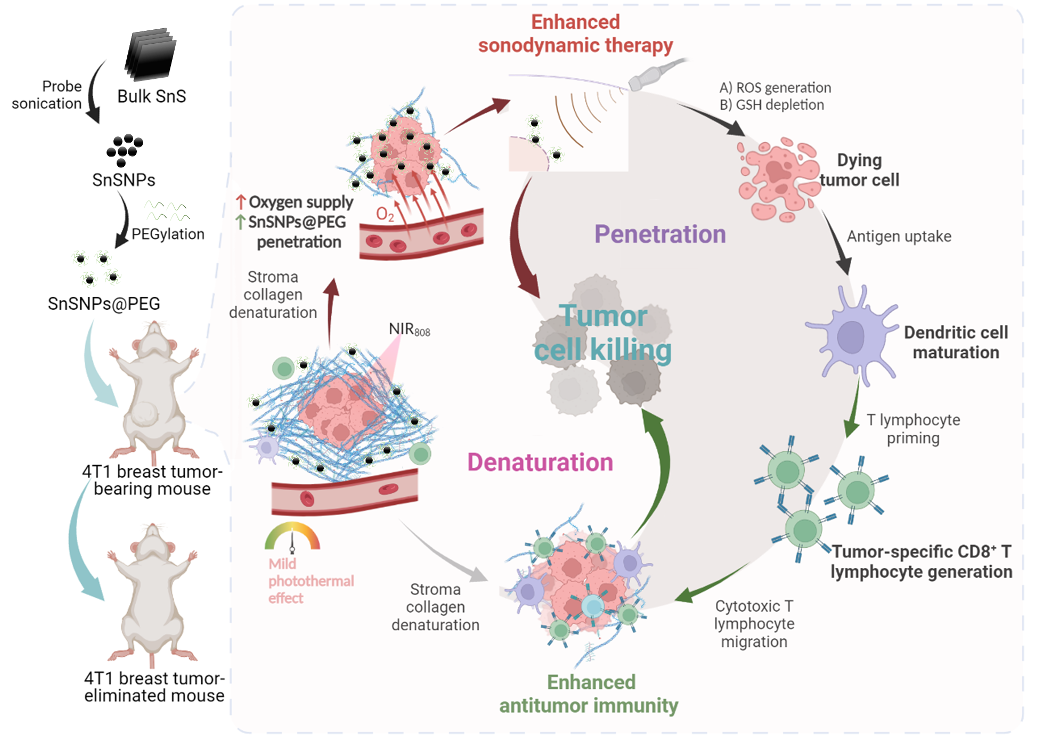
Nanosensitizer-mediated augmentation of sonodynamic therapy efficacy and antitumor immunity
The diffusion of nanomedicine and the infiltration of lymphocytes into tumors has been challenging due to the tumor stromal barrier. To overcome the barrier and optimize the therapeutic effect, we take the advantage of noninvasive in situ activable sonosensitizer and design a nanoparticle-mediate denaturation-and-penetration strategy to enhance SDT and antitumor immunity.
In this work, we designed an innovative denaturation-and-penetration strategy and developed SnSNPs as a nano-sonosensitizer to enhance sonodynamic therapy and antitumor immunity. Notably, this strategy markedly increased the intratumoral accumulation of SnSNPs by overcoming the tumor stromal barrier, enabled by the mild photothermal properties of SnSNPs. The SnSNP-based therapeutic approach demonstrated robust antitumor efficacy in orthotopic mouse models of triple-negative breast cancer (TNBC) and hepatocellular carcinoma (HCC), achieving noninvasive tumor eradication through efficient reactive oxygen species generation, immune activation, and enhanced infiltration of tumor-specific cytotoxic T lymphocytes, which collectively contributed to durable tumor control. [link]
Nature Communications 2023, 14(1):6973.
Cancer Sonodynamic-Immunotherapy
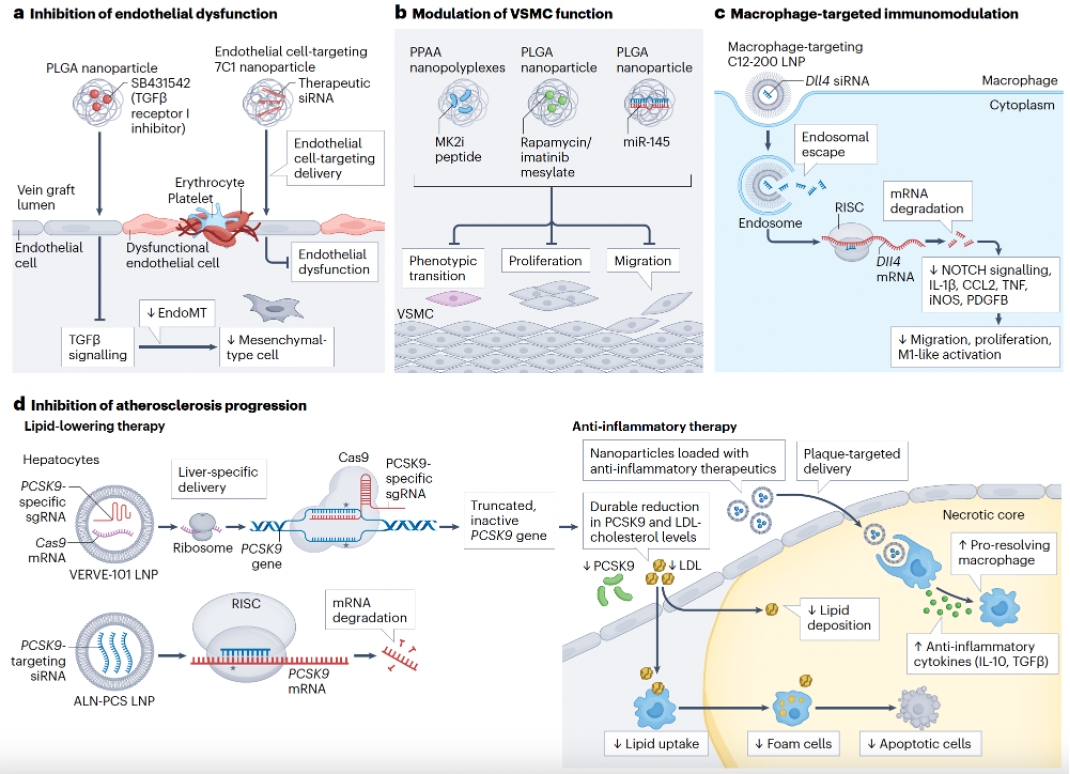
Nanomedicine-based Strategies for the Treatment of Vein Graft Disease
Currently, only a few therapeutic approaches for vein graft disease have been successfully translated into clinical practice. Building on the past two decades of advanced understanding of vein graft biology and the pathophysiological mechanisms underlying vein graft disease, nanomedicine-based strategies offer promising opportunities to address this important unmet clinical need.
In this Review, we provides deep insight into recent developments in the rational design and applications of nanoparticles that have the potential to target specific cells during various pathophysiological stages of vein graft disease, including early endothelial dysfunction, intermediate intimal hyperplasia, and late-stage accelerated atherosclerosis. Additionally, we underscore the convergence of nanofabricated biomaterials, with a particular focus on hydrogels, external graft support devices, and cell-based therapies, alongside bypass surgery to improve local delivery efficiency and therapeutic efficacy. [link]
Nature Reviews Cardiology 2025, 22(4):255-272.
Nanomedicine in Vein Graft Disease
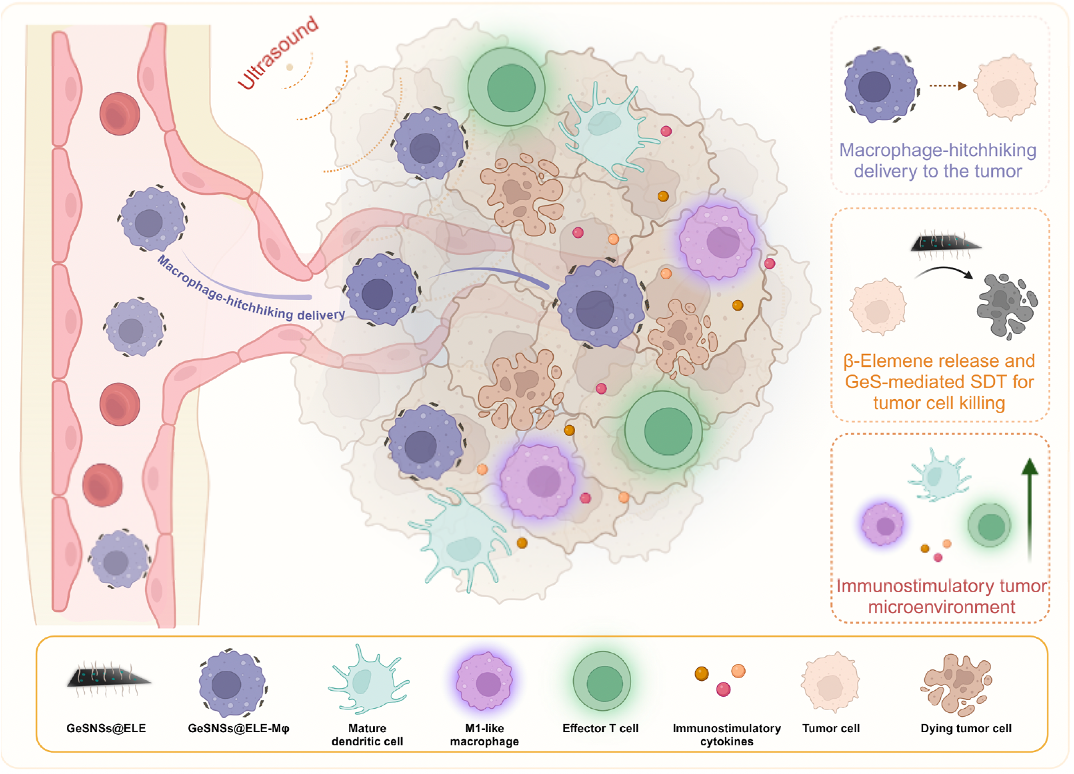
Macrophage hitchhiking nanomedicine for enhanced β-elemene delivery and tumor therapy
In recent years, nanoparticle-based drug delivery systems have emerged as promising strategies for cancer therapies. However, they still face challenges such as insufficient accumulation in lesions and suboptimal therapeutic efficacy following intravenous administration, primarily due to unwanted clearance by the reticuloendothelial system.
In this study, we used living macrophages as vehicles, attaching them with GeS nanosheets (GeSNSs) carrying β-elemene for transport to tumor sites. Notably, macrophage hitchhiking delivery of β-elemene–loaded GeSNSs not only achieves high accumulation in tumor regions and suppresses tumor growth under ultrasound treatment, but also effectively remodels the immunosuppressive tumor microenvironment, thereby facilitating enhanced sonodynamic chemoimmunotherapy. These findings underscore the potential of macrophage hitchhiking strategy for drug delivery and suggest broader applicability of engineered living materials–mediated delivery technologies in disease therapy. [link]
Science Advances 2025, 11(21):eadw7191.
Macrophage Hitchhiking Delivery Technology
Oral Delivery of Engineered Bacteria for Intestinal Disease Therapy
Orally deliverable strategy based on microalgal biomass for intestinal disease treatment
Oral drug delivery is the preferred and most commonly used route of drug administration for gastrointestinal (GI) disease treatment, mainly owing to its safety, high patient compliance, convenience, and ease of production. However, numerous challenges remain in oral drug delivery, such as the degradation of active pharmaceutical ingredients in the acidic environment of stomach, which results in their poor retention and low bioavailability in the intestine.
In this study, we report a versatile formulation based on a helical-shaped cyanobacterium, Spirulina platensis (SP), loaded with curcumin (SP@Curcumin) for the treatment of colon cancer and colitis, two types of intestinal diseases. The oral drug delivery system not only leveraged the biological properties of microalgal carriers to improve the bioavailability of loaded drugs but also performed excellent antitumor and anti-inflammation efficacy for intestinal disease treatment. [link]
Science Advances 2021, 7(48):eabi9265.
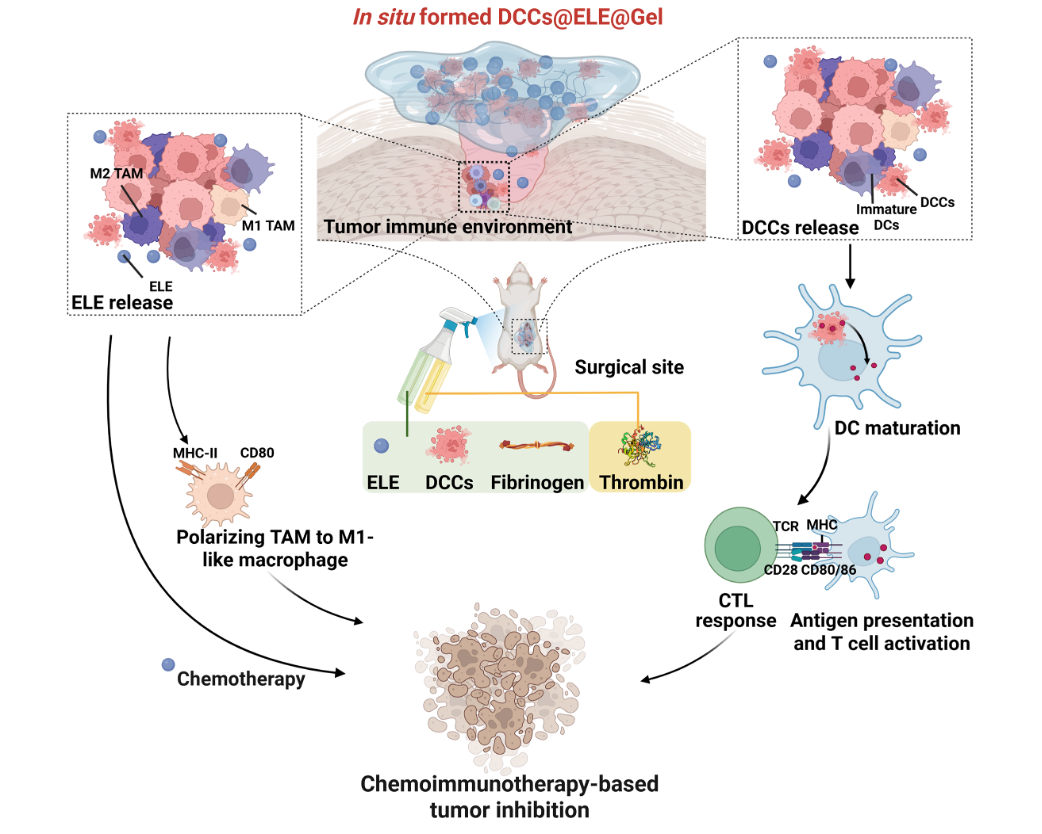
In-situ Sprayed Immunotherapeutic Hydrogel against Tumor Recurrence
“Cancer-treating-cancer” strategy: Entrapping engineered dying cancer cells in immunotherapeutic hydrogel against tumor recurrence
Postsurgical tumor recurrence remains a major challenge, primarily driven by the resurgence of residual microtumors at surgical margins. The tumor microenvironment (TME) in these regions plays a decisive role in treatment outcomes. Can the postsurgical tumor cavity be transformed from a permissive niche for tumor regrowth into an immunologically active site that educates long-term anti-tumor immunity?
In this study, we present a “cancer-treating-cancer” strategy by engineering dying cancer cells (DCCs) and combining them with β-elemene, a natural anticancer compound, within a fibrin-based hydrogel (DCCs@ELE@Gel). This hydrogel can form directly within the surgical cavity, where it adapts to the local tissue environment and serves as both a therapeutic depot and an immune stimulator. The engineered DCCs release tumor-associated antigens and immunogenic signals that stimulate the immune system, while β-elemene provides direct cytotoxic activity and enhances immunogenic cell death. Together, this combination remodels the tumor microenvironment, promotes dendritic cell maturation, activates cytotoxic T lymphocytes, and leads to the generation of memory T cells for long-term protection against relapse. This strategy highlights the translational potential of biomaterials to reprogram tumor-immune interactions and provides a new perspective for personalized postoperative cancer therapy. [link]
Cell Biomaterials 2025, 100252
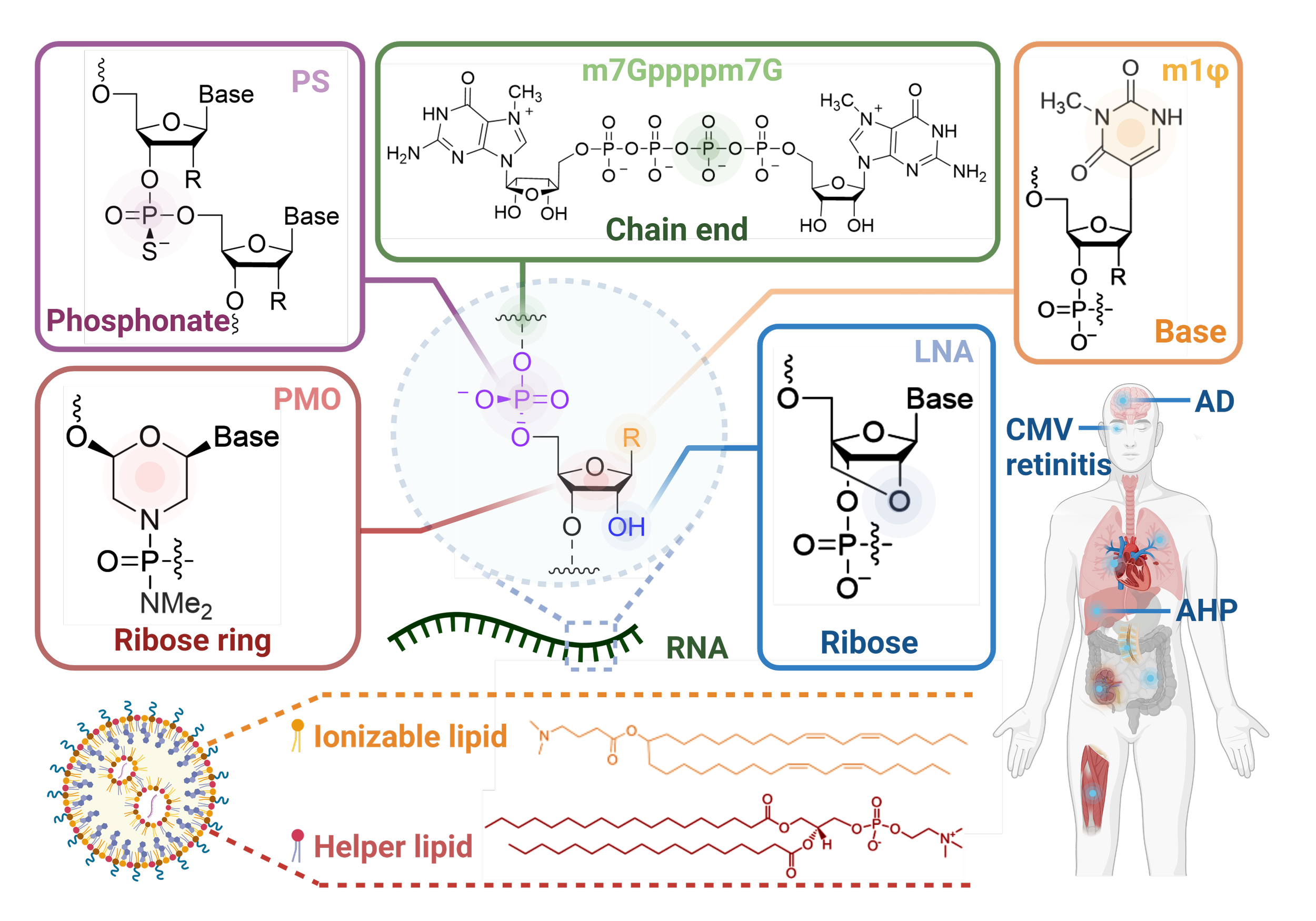
RNA Medicine for Disease Therapy
Chemically Modified Platforms for Better RNA Therapeutics
RNA-based therapies have catalyzed a revolutionary transformation in the biomedical landscape, offering unprecedented potential in disease prevention and treatment. However, despite their remarkable achievements, these therapies encounter substantial challenges including low stability, susceptibility to degradation by nucleases, and a prominent negative charge, thereby hindering further development.
Chemically modified platforms have emerged as a strategic innovation, focusing on precise alterations either on the RNA moieties or their associated delivery vectors. This comprehensive review delves into these platforms, underscoring their significance in augmenting the performance and translational prospects of RNA-based therapeutics (siRNA, microRNA, mRNA, ASO, and aptamer). [link]
Chemical Reviews 2024 Feb 14;124(3):929-1033.
Macrophage-Mediated Cancer Immunotherapy
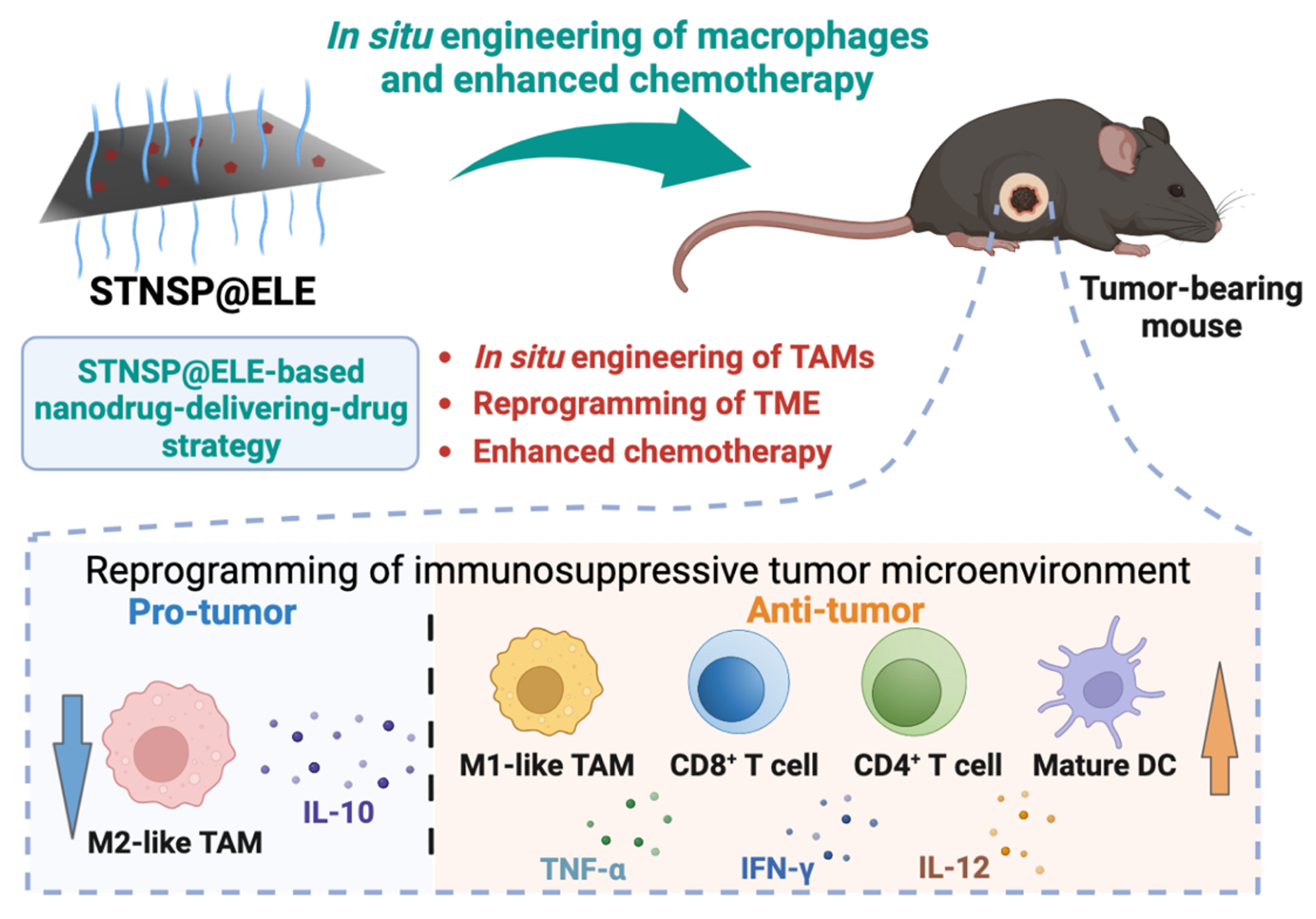
In situ Engineering of Tumor-Associated Macrophages via a Nanodrug-Delivering-Drug (β-Elemene@Stanene) Strategy for Enhanced Cancer Chemo-Immunotherapy
Despite the significant progress in clinical cancer immunotherapy, only a portion of patients with solid tumors respond to such therapy due to the presence of an immunosuppressive tumor microenvironment (TME), resulting in poor clinical outcomes and prognoses. One especially critical player in the TME is immunosuppressive tumor-associated macrophages (TAMs), which can induce robust tumor-localized immunosuppressive effects. However, generating tumor-suppressive M1-like TAMs through in situ engineering of M2-like TAMs for enhanced tumor immunotherapy remains a significant challenge in translational immuno-oncology.
In this study, we report an innovative nanodrug-delivering-drug (STNSP@ELE) strategy that leverages two-dimensional (2D) stanene-based nanosheets (STNSP) and β-Elemene (ELE), a small-molecule anticancer drug, to overcome TAM-mediated immunosuppression and improve chemo-immunotherapy. Our results demonstrate that both STNSP and ELE are capable of polarizing the tumor-supportive M2-like TAMs into a tumor-suppressive M1-like phenotype, which acts with the ELE chemotherapeutic to boost antitumor responses. In vivo mouse studies demonstrate that STNSP@ELE treatment can reprogram the immunosuppressive TME by significantly increasing the intratumoral ratio of M1/M2-like TAMs, enhancing the population of CD4+ and CD8+ T lymphocytes and mature dendritic cells, and elevating the expression of immunostimulatory cytokines in B16F10 melanomas, thereby promoting a robust antitumor response. [link]
Angewandte Chemie International Edition 2023, 62(41):e202308413.
(Highlighted as Top 5% Very Important Paper, VIP and Front Cover Paper)
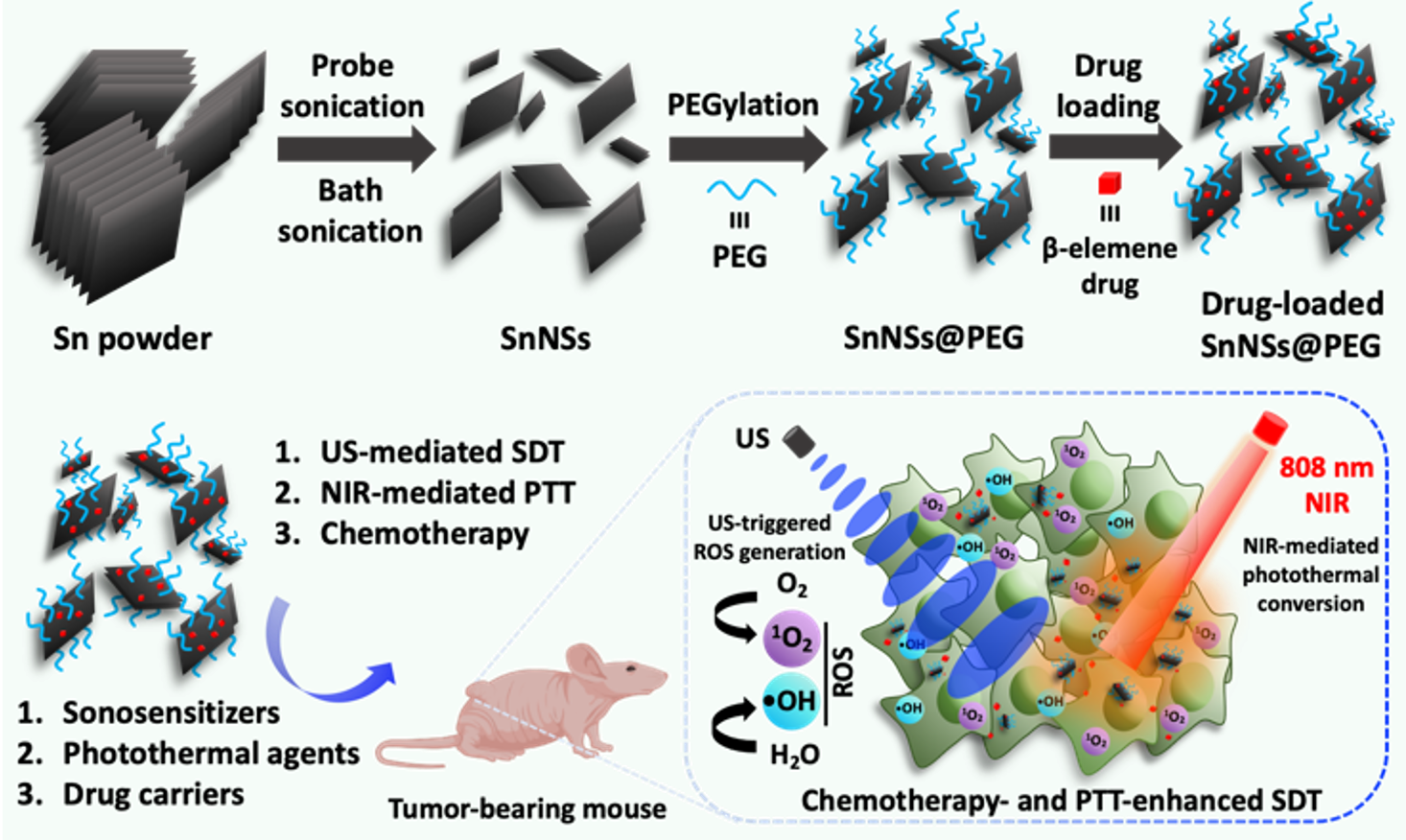
Stanene-Based Nanosheets for β-Elemene Delivery and Ultrasound-Mediated Combination Cancer Therapy
Ultrasound (US)-mediated sonodynamic therapy (SDT) has emerged as a superior modality for cancer treatment owing to the non-invasiveness and high tissue-penetrating depth. However, developing biocompatible nanomaterial-based sonosensitizers with efficient SDT capability remains challenging.
In this study, we report two-dimensional stanene-based nanosheets (SnNSs), prepared via a liquid-phase exfoliation strategy, as a new class of sonosensitizers for ultrasound-mediated SDT. Beyond their sonodynamic activity, SnNSs also function as efficient photothermal agents and versatile nanocarriers for β-elemene delivery, enabling photothermal therapy (PTT)–assisted combination treatment. This work not only establishes a scalable approach for the synthesis of SnNSs, but also introduces a general nanoplatform for tri-modal combination cancer therapy. [link]
Angewandte Chemie International Edition 2021, 60(13):7155-7164.
(Highlighted as Top 5% Very Important Paper, VIP and Front Cover Paper)
Ultrasound-Mediated Combination Cancer Therapy
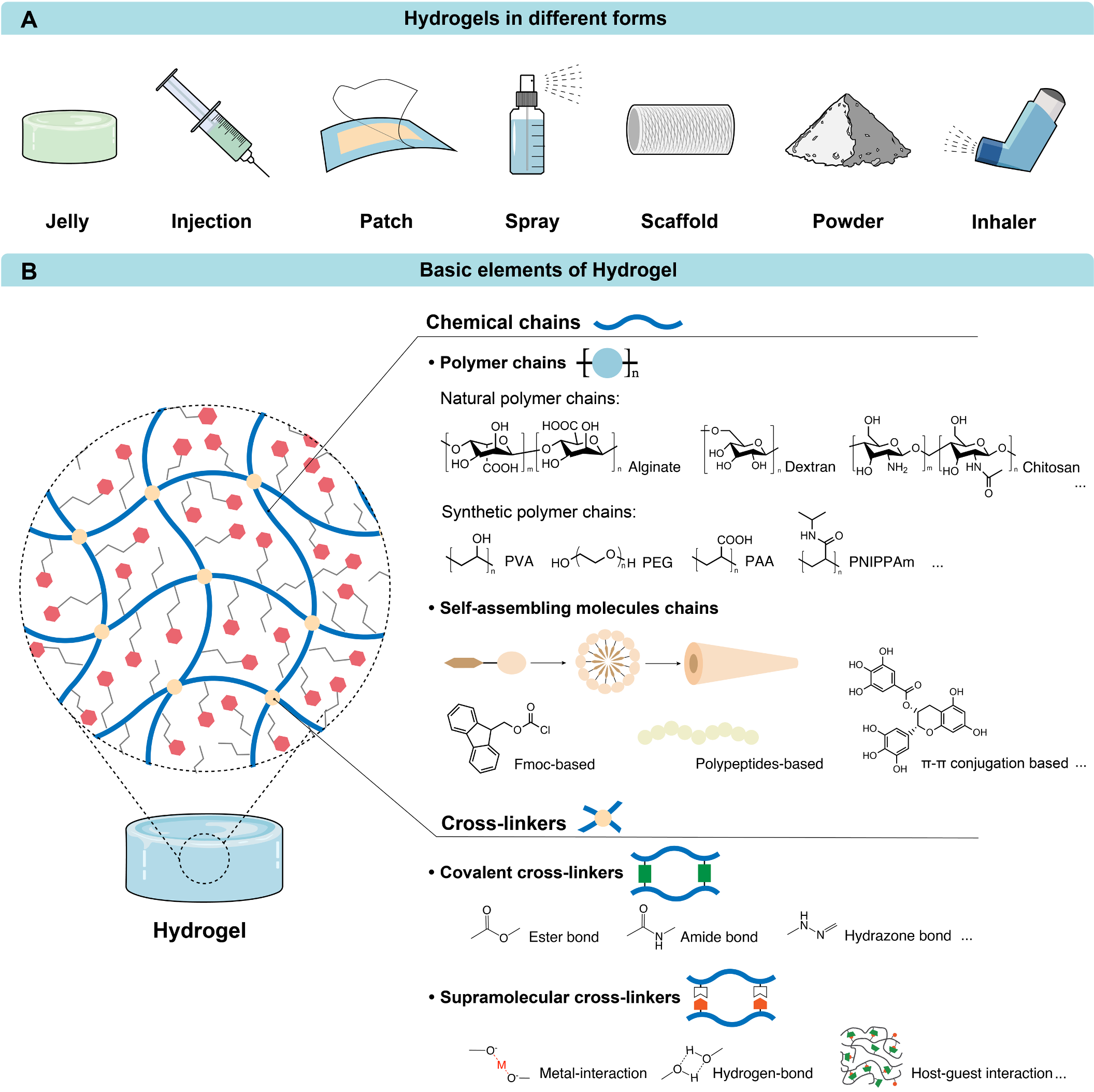
Therapeutic Hydrogels: Properties and Biomedical Applications
Hydrogels have emerged as versatile therapeutic platforms with immense potential for treating various diseases, due to their tunable properties and biocompatibility. Recent innovations, including injectable, self-assembling, and bioadhesive hydrogels, have broadened their biomedical applications, driven by advancements in materials chemistry.
In this review, we systematically examine the role of chemical principles in designing and customizing therapeutic hydrogels, with a focus on hydrogelation mechanisms, swelling ratios, mechanical properties, and biological interactions. By highlighting key studies in this field, this review explores how chemical chain modifications, cross-linking strategies, and cargo delivery systems have been tailored to achieve diverse functions, such as drug depots, wound dressings, antiadhesive barriers, and regenerative scaffolds. Addressing the gap in comprehensive analyses, this review underscores the integration of chemical design principles to optimize hydrogel properties for targeted therapies and discusses future opportunities to advance therapeutic hydrogel technology for a wide range of biomedical applications. [link]
Chemical Reviews 2025, 125(18):8835-8920.
(Highlighted as Cover Paper)
Therapeutic Hydrogels for Diverse Biomedical Applications
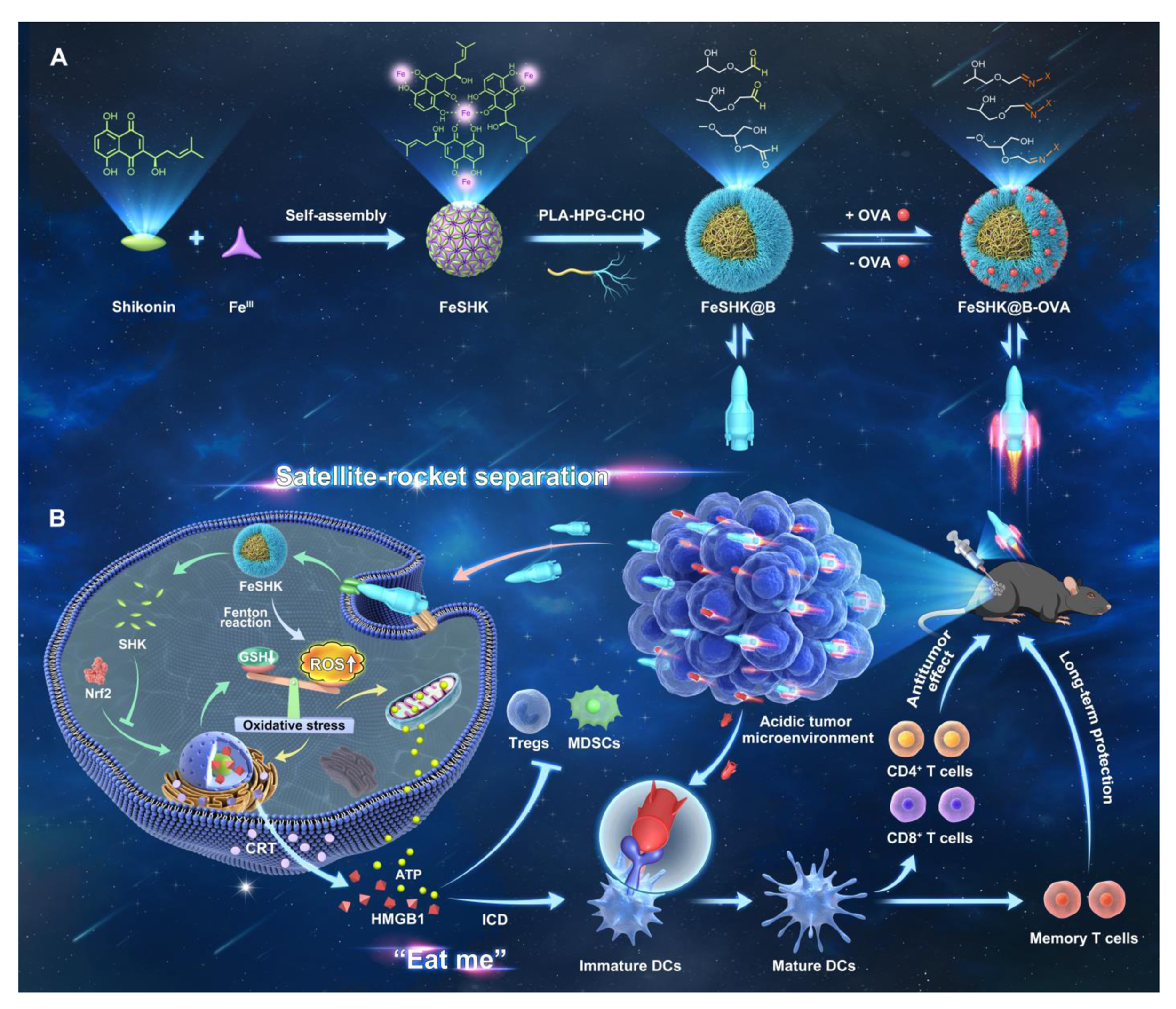
In situ separable nanovaccines with stealthy bioadhesivecapability for durable cancer immunotherapy
Tumor heterogeneity and the immunosuppressive tumor microenvironment severely limit the efficacy of cancer vaccines, resulting in weak antitumor immune responses in clinical trials. Although cancer immunotherapies—including immune checkpoint blockade, adoptive T-cell therapy, and monoclonal antibody–based approaches—have demonstrated remarkable potential, their clinical impact is often constrained by limited response rates, immune-related adverse events, and treatment-associated toxicities. These challenges underscore an urgent need for safer and more effective cancer immunotherapeutic strategies.
This study highlights the enormous potential of FeSHK@B-OVA, an in situ separable nanovaccinewith stealthy bioadhesive capability, to serve as an excellent therapeutic and prophylactic cancer nanovaccine. By leveraging the antigen depots in situ and the synergistic effect among multi-epitope antigens, such a nanovaccine strategy with stealthy bioadhesion may offer a straightforward and efficient approach to developing various cancer vaccines for different types of tumors. [link]
Journal of the American Chemical Society 2023, 145(15):8375–8388.
(Highlighted as Cover Paper)
Bioadhesive Cancer Nanovaccines
Near-Infrared II Bio-imaging
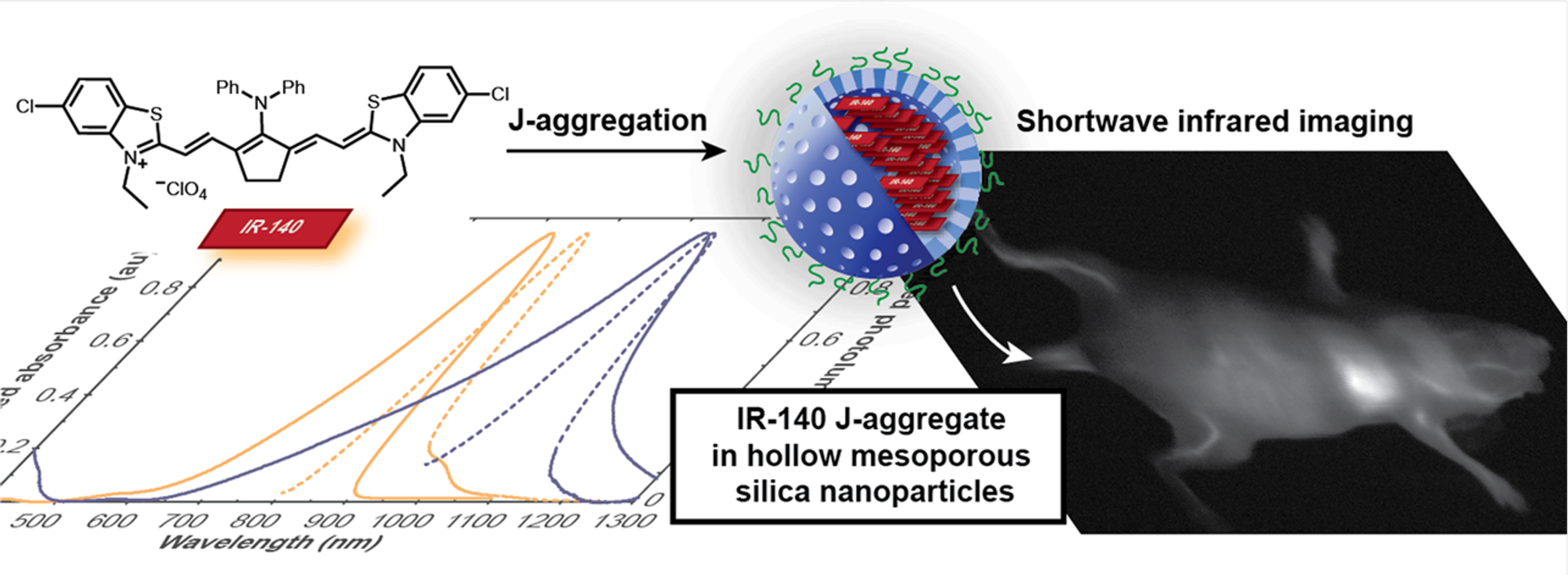
Shortwave Infrared Imaging with J-Aggregates Stabilized in Hollow Mesoporous Silica Nanoparticles
Tissue is translucent to shortwave infrared (SWIR) light, rendering optical imaging superior in this region. However, the widespread use of optical SWIR imaging has been limited, in part, by the lack of bright, biocompatible contrast agents that absorb and emit light above 1000 nm. J-Aggregation offers a means to transform stable, near-infrared (NIR) fluorophores into red-shifted SWIR contrast agents.
In this study, we demonstrate that J-aggregates of NIR fluorophore IR-140 can be prepared inside hollow mesoporous silica nanoparticles (HMSNs) to result in nanomaterials that absorb and emit shortwave infrared light. The J-aggregates inside PEGylated HMSNs are stable for multiple weeks in buffer and enable high resolution imaging in vivo with 980 nm excitation. [link]
Journal of the American Chemical Society 2019, 141(32):12475-12480.
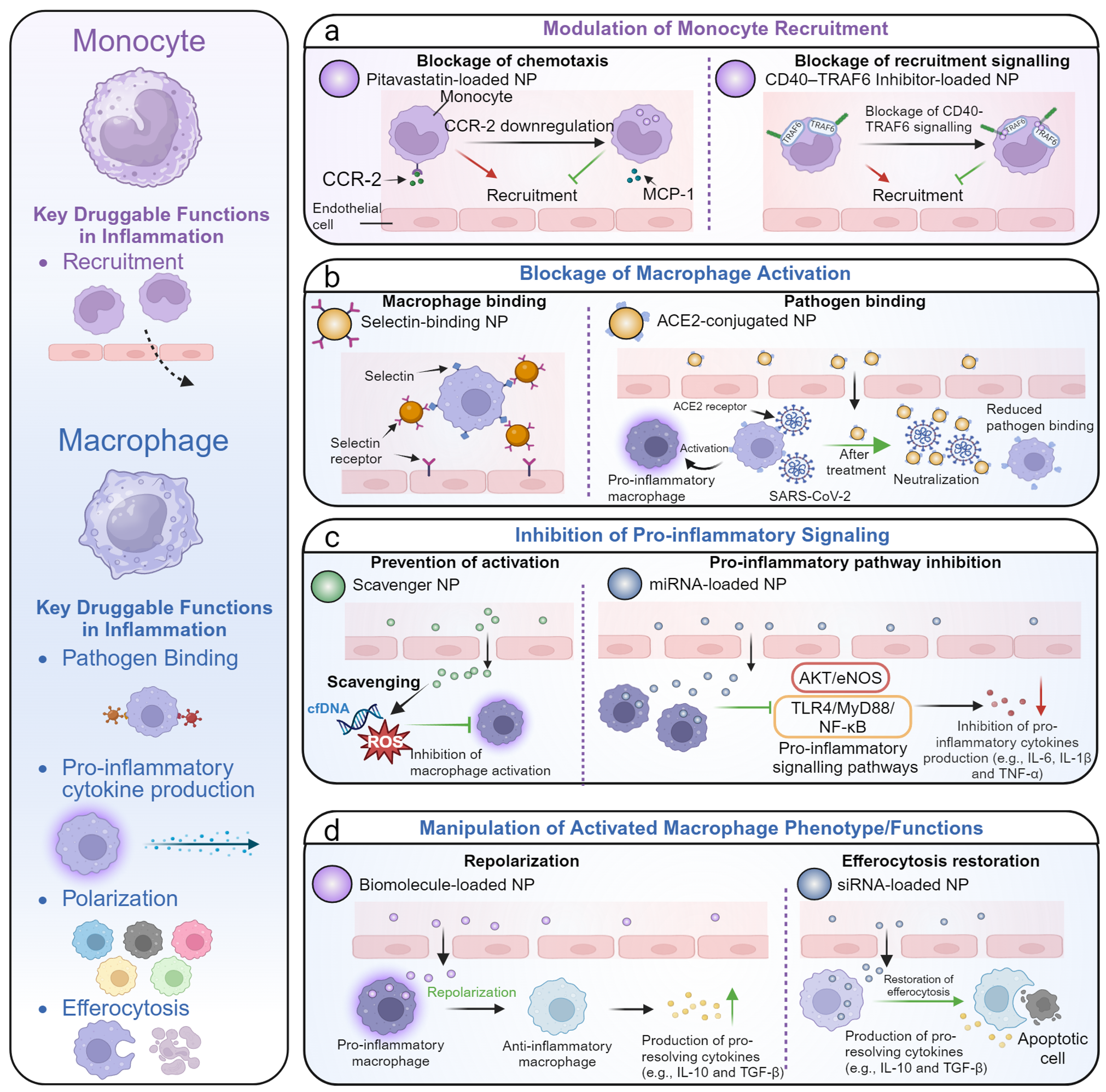
Innate immunity-modulating nanobiomaterialsfor controlling inflammation-resolution
The acute inflammatory response is an inherent protective mechanism, and its unsuccessful resolution can contribute to disease pathogenesis and potentially lead to death. Innate immune cells are the first line of host defenders and play a substantial role in inflammation initiation, amplification, resolution, or subsequent disease progression. As the resolution of inflammation is an active and highly regulated process, modulating innate immune cells, including neutrophils, monocytes, and macrophages, and endothelial cells, and their interactions offer opportunities to control excessive inflammation.
Here, we review innovative nanobiomaterials for modulating innate immunity and alleviating inflammation. We summarize strategies combining the design of nanobiomaterialswith the nano-bio interaction for modulating innate immune profiles and propelling the advancement of nanobiomaterials for inflammatory disease treatments. We also propose the future perspectives and translational challenges of nanobiomaterials that need to be overcome in this swiftly rising field. [link]
Matter (Cell Press) 2024, 7(11):3811-3844.
Nanobiomaterialsfor controlling inflammation resolution
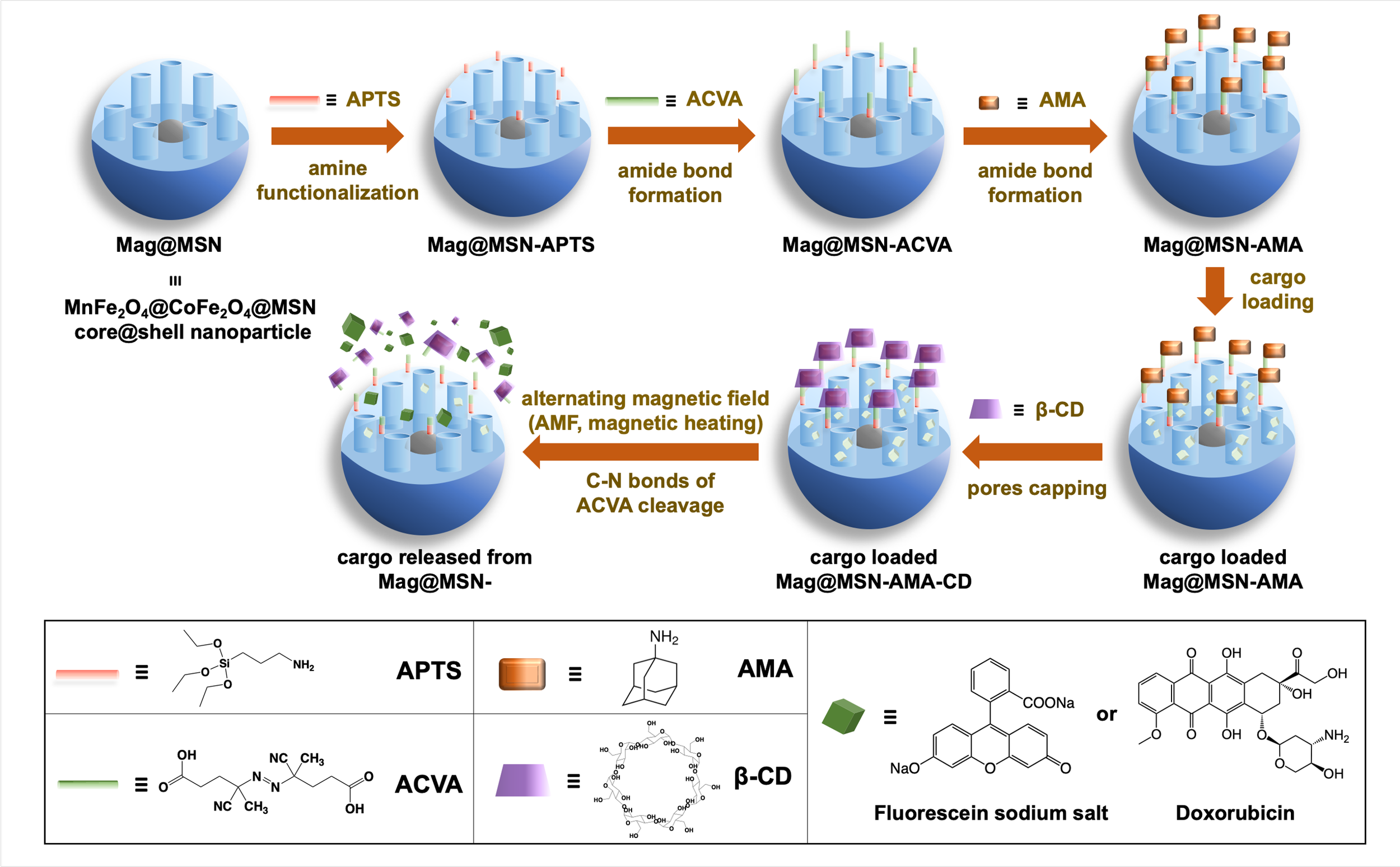
Spatial, Temporal, and Dose Control of Drug Delivery using Noninvasive Magnetic Stimulation
Precision medicine, defined as delivering the right drug at the right dose and time to the right patient, represents a rapidly advancing paradigm in cancer therapy. However, achieving precise, on-demand control over drug release dosage in vivo remains a major challenge for conventional delivery systems.
In this study, we report a stimuli-responsive nanoparticle platform that enables quantitative control of cargo release through actuation by an alternating magnetic field (AMF). This noninvasive magnetic stimulation strategy provides precise spatial, temporal, and dose control over therapeutic delivery, highlighting its strong potential for next-generation precision medicine applications. [link]
ACS Nano 2019, 13(2):1292-1308.
Stimuli-Responsive Drug Delivery System
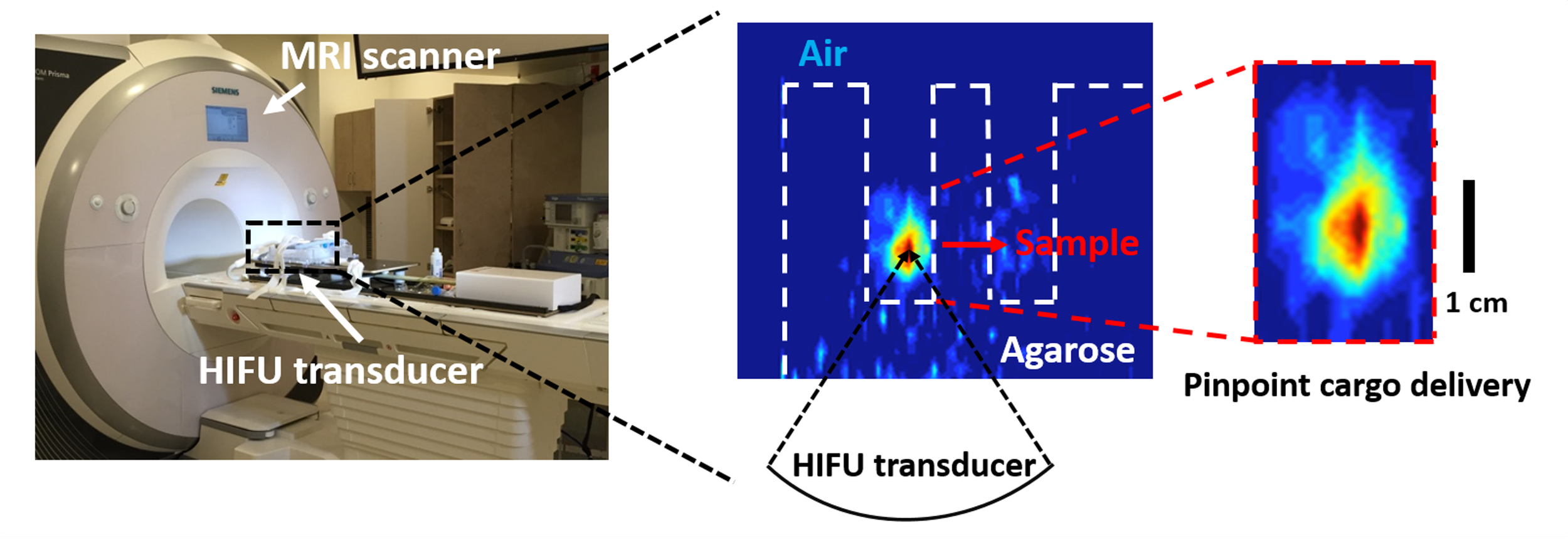
A Responsive Mesoporous Silica Nanoparticle Platform for Magnetic Resonance Imaging-Guided High-Intensity Focused Ultrasound-Stimulated Cargo Delivery with Controllable Location, Time, and Dose
Magnetic resonance imaging (MRI) is an essential clinical diagnostic modality, and MRI-guided high-intensity focused ultrasound (MRgHIFU) is a powerful technology for targeted therapy. Current clinical applications of MRgHIFU primarily rely on hyperthermia or thermal ablation to eradicate tumor tissue; however, such thermal effects are undesirable for drug delivery applications.
In this study, we report a biocompatible MRgHIFU-responsive mesoporous silica nanoparticle (MSN) platform that operates within a physiologically safe temperature range, thereby minimizing the risk of thermal damage to surrounding healthy tissues. This MRI-guided HIFU strategy enables real-time tracking of nanocarrier biodistribution and precise spatial control of ultrasound-triggered cargo release, opening new opportunities for image-guided theranostic applications. [link]
Journal of the American Chemical Society 2019, 141(44):17670-17684.
Imaging-Guided Drug Delivery Technology
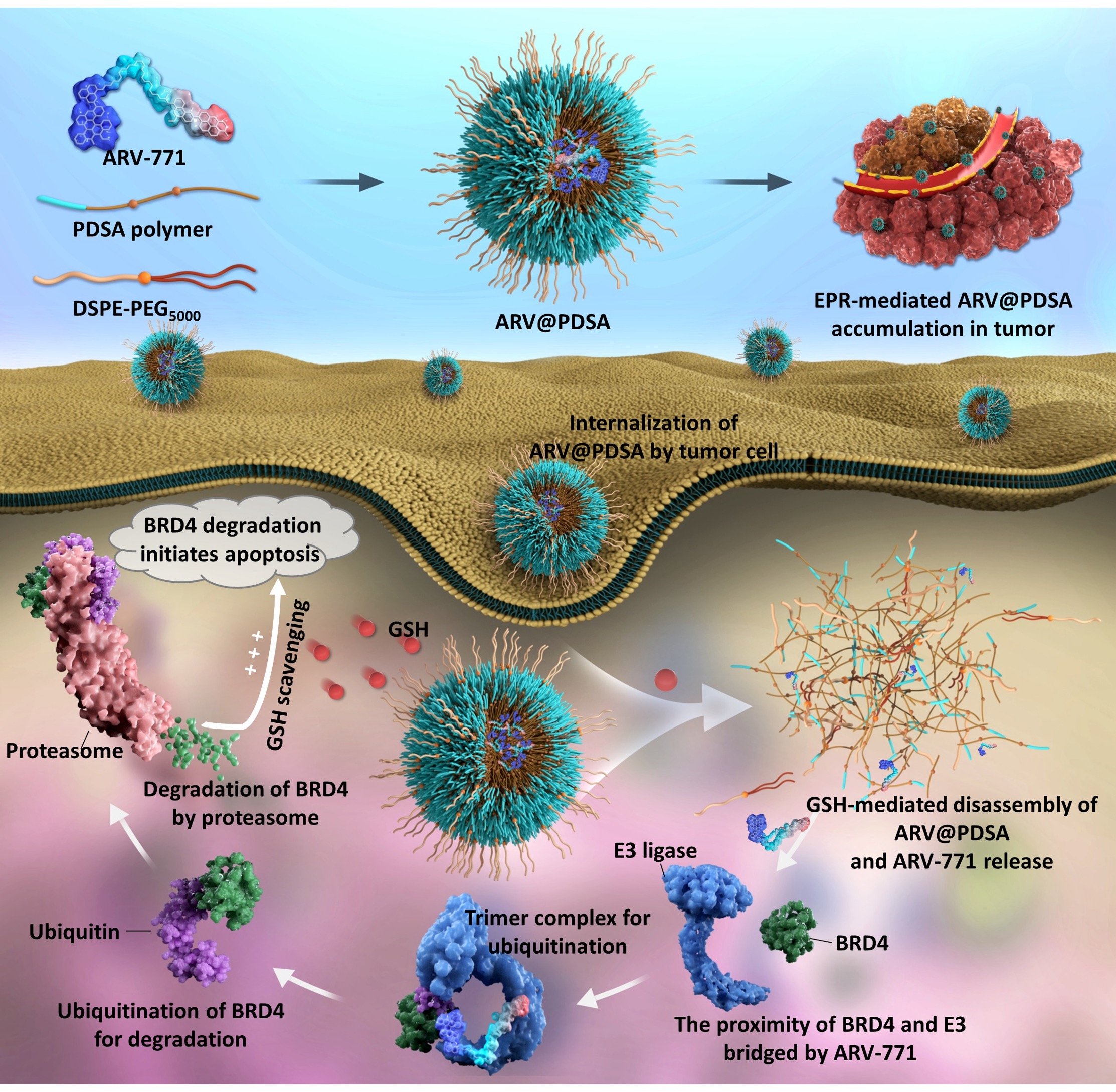
Glutathione-Scavenging Nanoparticle-Mediated PROTACs Delivery for Targeted Protein Degradation and Amplified Antitumor Effects
PROteolysis TArgeting Chimeras (PROTACs) are an emerging class of promising therapeutic modalities that selectively degrade intracellular proteins of interest by hijacking the ubiquitin-proteasome system. However, the lack of techniques to efficiently transport these degraders to targeted cells and consequently the potential toxicity of PROTACs limit their clinical applications.
A strategy of nanoengineered PROTACs, that is, Nano-PROTACs, is reported, which improves the bioavailability of PROTACs and maximizes their capacity to therapeutically degrade intracellular oncogenic proteins for tumor therapy. The Nano-PROTACs improves BRD4 protein degradation and decreases the downstream oncogene c-Myc expression, thereby shows superior anti-tumor efficacy. The findings reveal the potential of the Nano-PROTACs strategy to treat a broad range of diseases by dismantling associated pathogenic proteins. [link]
Advanced Science 2023, 10(16):e2207439.
Nano-PROTAC Technology for Tumor Therapy
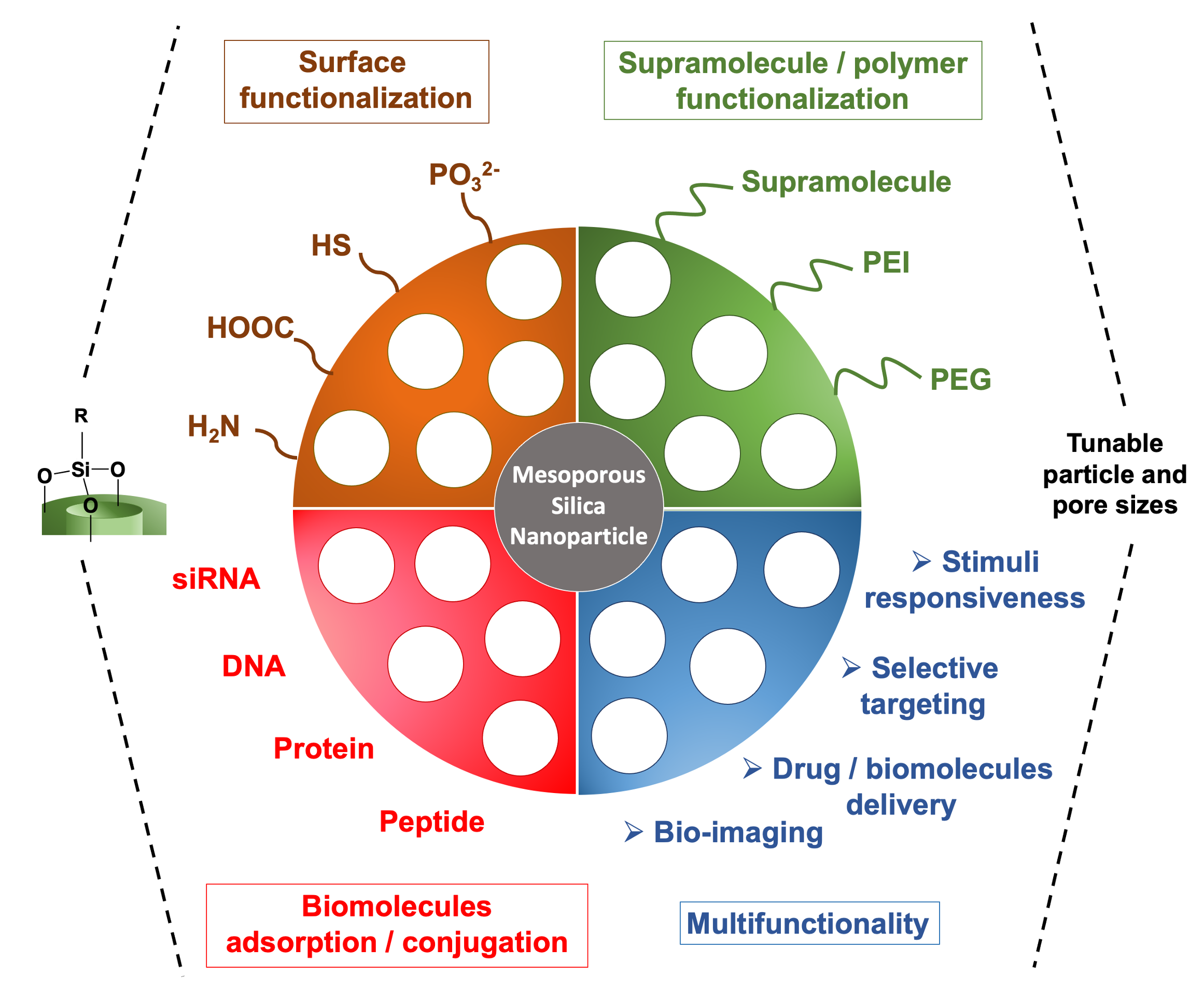
Nanomachines and Other Caps on Mesoporous Silica Nanoparticles for Drug Delivery
Mesoporous silica nanoparticles (MSNs) are delivery vehicles that can carry cargo molecules and release them on command. The particles used in the applications reported in this Account are around 100 nm in diameter (about the size of a virus) and contain 2.5 nm tubular pores with a total volume of about 1 cm3/g. For the biomedical applications discussed here, the cargo is trapped in the pores until the particles are stimulated to release it. The challenges are to get the particles to the site of a disease and then to deliver the cargo on command. We describe methods to do both, and we illustrate the applicability of the particles to cure cancer and intracellular infectious disease.
In this Account, we summarize the versatile applications of mesoporous silica as a drug delivery platform. These nanoparticles are non-toxic, capable of carrying substantial therapeutic drug payloads, and can be capped to prevent premature drug release. Upon reaching their target, they can be stimulated to release a high local concentration of the therapeutic agent. [link]
Accounts of Chemical Research 2019, 52(6):1531-1542.
Mesoporous Silica Nanoparticle for Drug Delivery
Selected Publications
Click for the full publication list: Google Scholar
2025
He Z, Luo Y, Duan Z, Su B, Zeng W, Guo Y, Li Y, He X, Shi H, Zhou Z, Jiang C, Qin D, Zhang J, Kang Y, Chen W*, Song X*. “IRF5 siRNA Nano-Immunotherapy: Restoring Macrophage Efferocytosis in Atherosclerosis.” Circulation 2025, 152, 1564–1581.
Zhou Z, Chen W*, Cao Y, Abdi R, Tao W*. “Nanomedicine-based Strategies for the Treatment of Vein Graft Disease.” Nature Reviews Cardiology 2025, 22, 255–272.
Chen S, Li Y, Zhou Z, Saiding Q, Zhang Y, An S, Khan MM, Ji X, Qiao R, Tao W*, Kong N*, Chen W*, Xie T*. “Macrophage hitchhiking nanomedicine for enhanced β-elemene delivery and tumor therapy.” Science Advances 2025, 11, adw7191.
Zhang Y, Zhou Z, Hao T, Chen S, Lou W, Khan MM, Shi Y, You X, Saiding Q, Zhen X, Tao W*, Tian Xie*, Chen W*, Kong N*. “Cancer-Treating-Cancer” Strategy: Entrapping Chemically Engineered Dying Cancer Cells in Immunotherapeutic Hydrogel Against Tumor Recurrence.” Cell Biomaterials 2025, 100252.
Dong S, An S, Saiding Q, Chen Q, Liu B, Kong N, Chen W*, Tao W*. “Therapeutic Hydrogels: Properties and Biomedical Applications.” Chemical Reviews 2025, 125, 8835–8920. (Cover Featured)
Liu C, Huang X, Chen K, Xiong S, Yaremenko A, Zhen X, You X, Rossignoli F, Tang Y, Koo S, Chen W, Kong N, Xie T, Shah K, Tao W*. “Systemic reprogramming of tumour immunity via IL-10-mRNA nanoparticles.” Nature Nanotechnology 2025, 20, 1526–1538.
Huang X, Liu C, Sharma S, Chen S, Li Y, Liu H, Liu B, Saiding Q, Chen W, Lee Y, Kong N, Abdi R, Tao W*. “Oral delivery of liquid mRNA therapeutics by an engineered capsule for treatment of preclinical intestinal disease.” Science Translational Medicine 2025, 17, adu1493.
2024
He Z#, Chen W#*, Hu K#, Luo Y, Zeng W, He X, Li T, Ouyang J, Li Y, Xie L, Zhang Y, Xu Q, Yang S, Guo M, Zou W, Li Y, Huang L, Chen L, Zhang X, Saiding Q, Wang R, Zhang MR, Kong N, Xie T, Song X,* Tao W*. “Resolvin D1 delivery to lesional macrophages using antioxidative black phosphorus nanosheets for atherosclerosis treatment.” Nature Nanotechnology 2024, 19, 1386–1398.
(Highlighted in the “Biomedical Engineering” Series of articles from across Nature Portfolio) (Top 1% ESI Highly Cited Paper)Shi Y, Zhen X, Zhang Y, Li Y, Koo S, Saiding Q, Kong N, Liu G, Chen W*, Tao W*. “Chemically Modified Platforms for Better RNA Therapeutics.” Chemical Reviews 2024, 124, 929–1033. (Top 1% ESI Highly Cited Paper)
Li Y#, Chen W#, Koo S, Liu H, Saiding Q, Xie A, Kong N, Cao Y, Abdi R, Serhan CN, Tao W*. “Innate immunity-modulating nanobiomaterials for controlling inflammation.” Matter (Cell Press) 2024, 7, 3811-3844.
Khan MM, Li Y, Zhou Z, Ni A, Saiding Q, Qin D, Tao W*, Chen W* “Macrophage-modulating nanomedicine for cancer immunotherapy.” Nanoscale 2024, 16, 7378–7386. (Invited Article; Royal Society of Chemistry Nanoscale Emerging Investigators Collection)
2023
Chen W, Li Y, Liu C, Kang Y, Qin D, Chen S, Zhou J, Liu H, Ferdows BE, Patel DN, Huang X, Koo S, Kong N, Ji X, Cao Y, Tao W, Xie T “In situ Engineering of Tumor-Associated Macrophages via a Nanodrug-Delivering-Drug (β-Elemene@Stanene) Strategy for Enhanced Cancer Chemo-Immunotherapy.” Angew. Chem. Int. Ed. 2023, 62, e202308413.
(Highlight as Top 5%Very Important Paper VIP, Front Cover Featured, Top 1% ESI Highly Cited Paper, Top 0.1% ESI Hot Paper)Li Y#, Chen W#, Kang Y, Zhen X, Zhou Z, Liu C, Chen S, Huang X, Liu H, Koo S, Kong N, Ji X, Xie T, Tao W*. “Nanosensitizer-mediated augmentation of sonodynamic therapy efficacy and antitumor immunity.” Nature Communications 2023, 14, 6973.
(Top 1% ESI Highly Cited Paper, Highlighted in the "Biomedical applications for nanotechnologies" Collection)Yu L#, Yu M#, Chen W#, Sun S, Huang W, Wang T, Peng Z, Luo Z, Fang Y, Li Y, Deng Y, Wu M, Tao W. “In situ separable nanovaccines with stealthy bioadhesive capability for durable cancer immunotherapy.” J. Am. Chem. Soc. 2023, 145, 8375–8388. (Cover Paper, Top 1% ESI Highly Cited Paper)
Liu H#, Chen W#, Wu G#, Zhou J, Liu C, Tang Z, Huang X, Gao J, Xiao Y, Kong N, Joshi N, Cao Y, Abdi R, Tao W*. “Glutathione-scavenging nanoparticle-mediated PROTACs delivery for targeted protein degradation and amplified antitumor effects.” Advanced Science 2023, 10, 2207439.
Ma S#, Kim JH#, Chen W#, Li L, Lee J, Xue J, Liu Y, Chen G, Tang B, Tao W, Kim JS “Cancer Cell-Specific Fluorescent Prodrug Delivery Platforms.” Advanced Science 2023, 10, 2207768
Nie Y#, Chen W#, Kang Y#, Yuan X, Li Y, Zhou J, Tao W, Ji X “Two-dimensional porous vermiculite-based nanocatalysts for synergetic catalytic therapy.” Biomaterials 2023, 295, 122031.
Chen S, Huang X, Xue Y, Álvarez-Benedi E, Shi Y, Chen W, Koo S, Siegwart D, Dong Y, Tao W* “Nanotechnology-based mRNA vaccines.” Nature Reviews Methods Primers 2023, 3, 63. (Highlighted in the “Nobel Prize in Physiology or Medicine 2023” Collection)
2022
Chen W, Schilperoort M, Cao Y, Shi J*, Tabas I*, Tao W* “Macrophage-Targeted Nanomedicine for the Diagnosis and Treatment of Atherosclerosis.” Nature Reviews Cardiology 2022, 19, 228–249.
(Front Cover Featured; Top 1% ESI Highly Cited Paper; Highlighted in the "Mechanisms of atherosclerosis" Series of articles from Nature Reviews Cardiology)Chen W, Tao W* “Precise Control of the Structure of Synthetic Hydrogel Networks for Precision Medicine Applications.” Matter (Cell Press) 2022, 5, 18–19.
Ouyang J, Chen W*, Tao W* “Design of application-optimized hydrogels based on a swollen polymer network model.” Matter (Cell Press), 2022, 5, 2471–2473.
Zhang D, Zhong D, Ouyang J, He J, Qi Y, Chen W, Zhang X, Tao W, Zhou M “Microalgae-based oral microcarriers for gut microbiota homeostasis and intestinal protection in cancer radiotherapy.” Nature Communications 2022, 17, 1413. (Top 1% ESI Highly Cited Paper)
2021
Chen W, Liu C, Ji X, Joseph J, Tang Z, Ouyang J, Xiao Y, Kong N, Joshi N, Farokhzad OC, Tao W, Xie T “Stanene-Based Nanosheets for β-Elemene Delivery and Ultrasound-Mediated Combination Cancer Therapy.” Angew. Chem. Int. Ed. 2021, 60, 7155–7164.
(Highlight as Top 5% Very Important Paper VIP, Front Cover Featured, Top 1% ESI Highly Cited Paper)Zhong D#, Zhang D#, Chen W#, He J, Ren C, Zhang X, Kong N, Tao W, Zhou M “Orally Deliverable Strategy Based on Microalgal Biomass for Intestinal Disease Treatment.” Science Advances 2021, 7, abi9265.
(Front Cover Paper, Top 1% ESI Highly Cited Paper) (Highlighted: Nature Medicine 2022, 28(6): 1100-1102)Ji X, Ge L, Liu C, Tang Z, Xiao Y, Chen W, Lei Z, Gao W, Blake S, De D, Shi B, Zeng X, Kong N, Zhang X, Tao W. “Capturing Functional Two-Dimensional Nanosheets from Sandwich-Structure Vermiculite: Synthesis and Application in Cancer Theranostics.” Nature Communications 2021, 12, 1124. (Web of Science Top 0.1% Hot Paper | Top 1% ESI Highly Cited Paper)
2019
Chen W, Cheng CA, Cosco ED, Ramakrishnan S, Lingg JGP, Bruns OT*, Zink JI*, Sletten EM*. “Shortwave Infrared Imaging with J-aggregates Stabilized in Hollow Mesoporous Silica Nanoparticles.” J. Am. Chem. Soc. 2019, 141, 12475–12480. (Top 1% ESI Highly Cited Paper)
Chen W, Cheng CA, Zink JI*. “Spatial, Temporal, and Dose Control of Drug Delivery Using Noninvasive Magnetic Stimulation.” ACS Nano 2019, 13, 1292–1308. (Top 1% ESI Highly Cited Paper)
Chen W, Glackin CA, Horwitz MA, Zink JI*. “Nanomachines and Other Caps on Mesoporous Silica Nanoparticles for Drug Delivery.” Acc. Chem. Res. 2019, 52, 1531–1542. (Top 1% ESI Highly Cited Paper)
Cheng CA#, Chen W#, Zhang L, Wu HH*, Zink JI*. “A Responsive Mesoporous Silica Nanoparticle Platform for Magnetic Resonance Imaging-Guided High-Intensity Focused Ultrasound-Stimulated Cargo Delivery with Controllable Location, Time, and Dose.” J. Am. Chem. Soc. 2019, 141, 17670–17684.
2018
Chen W, Cheng CA, Lee BY, Clemens DL, Huang WY, Horwitz MA, Zink JI*. “Facile Strategy Enabling Both High Loading and High Release Amounts of the Water-Insoluble Drug Clofazimine Using Mesoporous Silica Nanoparticles.” ACS Appl. Mater. Interfaces 2018, 7, 31870–31881.
Chen W, Tsai PH, Hung Y, Chiou SH*, Mou CY* “Nonviral Cell Labeling and Differentiation Agent for Induced Pluripotent Stem Cells Based on Mesoporous Silica Nanoparticles.” ACS Nano 2013, 7, 8423–8440.